Summary
Transcranial Laser Therapy (TLT) using a high-powered, near-infrared laser (HPNIL) has shown very impressive benefits to PTSD and TBI patients in a series of 10 patients. Those beneficial effects included reduced depression, reduced suicidal thoughts, reduced sleep disturbance, and increased employment. This study will treat 40 Veterans with PTSD using TLT, and simultaneously seeks to demonstrate that TLT at a four-fold higher dose can achieve better and faster PTSD treatments. This study will measure multiple outcomes at frequent intervals to better define the temporal and dosage relationships of TLT.
Although there are a number of treatments for PTSD, none routinely achieve more than 50% cure rates, and none can scale to treat the millions of patients with PTSD. Recent advances in commercial medical HPNIL devices and in neuroanatomical understanding of PTSD can be combined to show that TLT could theoretically be used at up to 10 times the dose previously found to be clinically beneficial. If clinical results from the Phase 1 studies demonstrate that clinical benefits are proportional to total TLT Joules, this would put TLT at the forefront of PTSD treatments, while also significantly shortening the treatment time, improving treatment results, and decreasing costs. This pilot Phase I study is a critical first step towards these goals.
Background
PTSD
PTSD is one of the largest problems facing Veterans, and PTSD-related suicide among Veterans is at an epidemic level. Less than 50% of Veterans with PTSD seek treatment. Current treatment options are effective less than 50% of the time and typically require months of treatment. These treatments require substantial active participation from the patient, which limits their applicability. Due to being labor-intensive, they cannot scale to treat the millions of people with PTSD, and are simultaneously restrained by high costs.
Neuroanatomy of PTSD & TLT
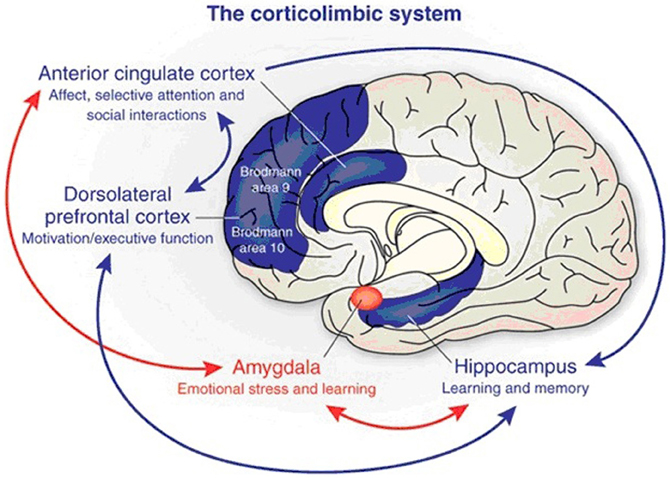
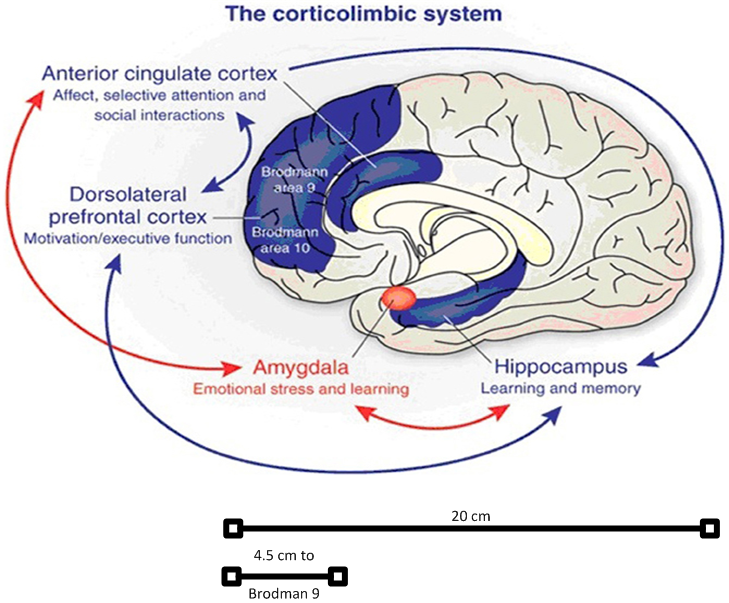
Over the past ten years, a multitude of studies have shown that PTSD causes specific functional and anatomic brain changes. Specifically, the prefrontal cortex (PFC) is hypoactive and its volume reduced, the amygdala is hyperactive, and the hippocampus has reduced volume. The anterior cingulate cortex may also be hyperactive.
The PFC, corresponding to Brodmann areas 9 and 10, is the primary target of TLT. The PFC helps planning complex cognitive and executive behaviors, personality expression, decision making, and moderating correct social behavior.
The Amygdala is the center of our emotions, and has a primary role in processing and memory of emotional reactions. The Amygdala is situated deep in the temporal lobe, in front of the hippocampus.
The hippocampus functions to control autonomic, emotional and sexual behavior. The hippocampus and amygdala are part of the limbic system, which is responsible for autonomic, emotional, sexual, and fight-or-flight behavior. The hippocampus plays an important role in long-term memory and may be involved in explicit memories of traumatic events.
The main working theory of PTSD is that there is an initial fight-or-flight survival response that occurs during the initiating stress. This activates the entire limbic system and especially the amygdala, and suppresses the PFC so that we may focus all of our energy on survival. Subsequently, most people's brains return to normal following a major stress. However, in PTSD, the amygdala remains hyperactive, and the PFC becomes hypoactive and physically shrinks. There is a 2 minute video at http://ww.care which succinctly illustrates this process, and the diagram below shows the corticolimbic system.
From: http://brucedowmd.com/wp-content/uploads/2013/05/amygdala-and-acc2.jpg
The goal and theory of TLT is to aim high-power near-infrared laser light directly into the skull. The target tissue is primarily Brodmann's 9 and 10, the PFC. The photons cross the skull and are absorbed by mitochondria in the neurons, stimulating ATP production and increasing neuronal activity and nerve growth factor. The increased PFC activity then suppresses the amygdala. Secondary target tissues are the fronto-temporal cortical areas associated with depression. The anterior cingulate cortex, hippocampus, and amygdala are not target tissues for direct laser stimulation
HPNIL Devices
Class IV lasers ( > 0.5 Watts) have become increasingly available in the last five years. Just within the last year, three high power near-infrared lasers have come on the market. These are FDA-cleared for treating somatic pain. The Litecure EXP provides up to 25 watts of combined 810 and 980 nm near-infrared light. Diowave offers an 810 and a 980 nm unit, each capable of up to 60 watts. For this study, the LMDPR clinic's LiteCure EXP 25 watt HPNIL device will be used. Additionally, it may be possible to acquire 1 or 2 Diowave 60-watt units for use in this study, and if so, they will be used for treating some patients.
HPNIL Transmission & Minimum Effective Dose
A number of questions need to be addressed in order to derive whether laser therapy, applied outside of the head at or near the scalp and directed towards the brain can achieve any kind of meaningful power inside of the brain. The basic parameters are power (Watts, W), time, energy (Joules, J = 1 watt x 1 second), pulse or continuous wave, transmission (or absorption or loss) and frequency.
The 810-980 nm frequency range is near-infrared and has been shown to achieve maximum penetration of human tissue compared with < 800 and > 1000 nm lasers. There is some indication that 980 nm may have better penetration while 810 nm may produce more biologic effects, but the data are not crystal clear.
Two studies in the past year have quantified transmission of near-infrared laser (NIL or NILT) through brain and skull. Henderson, in ex vivo lamb skull and brain, showed transmission approaching 1-2% of the initial power from at 3 cm from the scalp using a LiteCure and other HPNILs. Henderson's Denver Protocol article states "extensive research has shown the fluence within the range of 0.9–15.0 J/cm 2 is most effective in activating the biological processes involved in reversing or mitigating the pathophysiological effects of TBI." …. From our data, we estimate that in our clinical applications of high-powered NIR lasers, we are delivering 0.64–1.95 J/cm 2 to a depth of 30 mm." This was using a Litecure 10 W 810/980 nm or the Diowave 810 nm 15 W pulsed HPNIL. Combining both of Henderson's articles, and working backwards, it is possible to show the consistency of their calculations. Each treatment was approximately 20 minutes over 40-50 cm 2 . Litecure 10 W pulsed produced 0.0230 W at a distance of 3 cm, with a duration of 20 minutes (1200 seconds) this would produce 27J, and divide by 40 cm 2 to get fluence of 0.6 J/cm 2 . The same calculations for the Diowave 15W pulsed which according to their table 3 produced 0.0478 Watts, yields ~ 2 J/cm 2 .
A more elaborate and in-depth study of NIL transmission was recently published by Tedford et al using ex-vivo human skull and brain. Using 810 and 980 nm NIL, they found transmission of ~ 0.1% at 3 cm from the scalp, and ~ 0.01% at 4 cm from the scalp. Using Henderson's "extensive research has shown the fluence within the range of 0.9–15.0 J/cm 2 is most effective in activating the biological processes", we can work backwards to calculate that 10,000 J/cm 2 at the scalp is required to produce the minimum 1 J/cm 2 at 4 cm , and 150,000 J/cm 2 would yield the maximum quoted figure of 15 J/cm 2 . Of course, the fluence at 3 cm would be ~ 10X higher, so a reasonable middle approach would be to aim for 2,000 - 20,000 J/cm 2 at the scalp.
The Denver protocol produced meaningful PTSD treatment using a dose of 50,000 - 180,000 total Joules per treatment area totaling about 100 cm 2 , or about 500 - 1,800 J/cm 2 (all figures are based on the total Joules over the entire treatment cycle of 10-20 weeks.) Clearly, while this dose may be at the very lowest therapeutic range in the first 1-2 cm, it will be sub-therapeutic or sub-sub-therapeutic at 3-4 cm distance.
The Manhattan Beach protocol will use ~ 4X the dose, in a shorter time period. A dose of 472,500 J per treatment area totaling about 120 cm 2 , will yield about 4,000 J/cm 2 . While this is about 4X the therapeutic Joules and 2.5X the fluence of the Denver protocol, it may still be at the low end of maximum therapeutic dose.
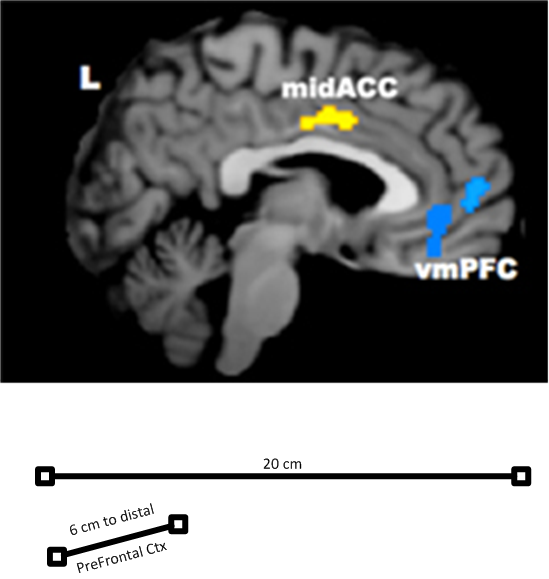
One potential danger of the higher doses is that scalp and even brain heating can occur. Therefore, this study employs continuous two-point scalp temperature monitoring and recording. Further studies to explore even higher doses will be utilized if clinical results from this study are positive. As can be seen in the following diagrams, based on a 20 cm long skull, it may take 4.5 - 6.0 cm to fully cover the distance from the scalp to the furthest reaches of the PFC.
https://upload.wikimedia.org/wikipedia/commons/6/61/HeadAnthropometry.JPG
|
Distance to brain, Laser Power at Scalp, Fluence in Brain, & Different Protocols |
|||||
|
Distance from skull to brain |
Range of Effective Doses |
NEST-3 |
"Denver Protocol" - Henderson |
"Manhattan Beach Protocol" - This study |
Future Manhattan Beach protocols |
|
Scalp |
To reach 1 J/cm 2 at 4 cm, need ~ 10,000 J/cm 2 at the scalp |
One session = 5W, 120 seconds, 100%, => 60 J/cm 2 |
Each session = 10 W, 1200 seconds, 50% pulsed, 20 sessions, => 1,800 J/cm 2 |
Each session = 25 W, 1200 seconds, 100% continuous, 15 sessions => 4,000 J/cm 2 |
Each session = 60 W, 1500 seconds, 100% continuous, 15 sessions =>12,000 J/cm 2 |
|
3 cm calculated |
|
0.06 J/cm 2 |
0.64–1.95 J/cm 2 |
4 J/cm 2 |
12 J/cm 2 |
|
4 cm calculated |
|
0.006 J/cm 2 |
0.06 - 0.2 J/cm 2 |
0.4 J/cm 2 |
1.2 J/cm 2 |
|
Brain dose for good effect |
0.9 - 15 J/cm 2 |
0.9 - 15 J/cm 2 |
0.9 - 15 J/cm 2 |
0.9 - 15 J/cm 2 |
0.9 - 15 J/cm 2 |
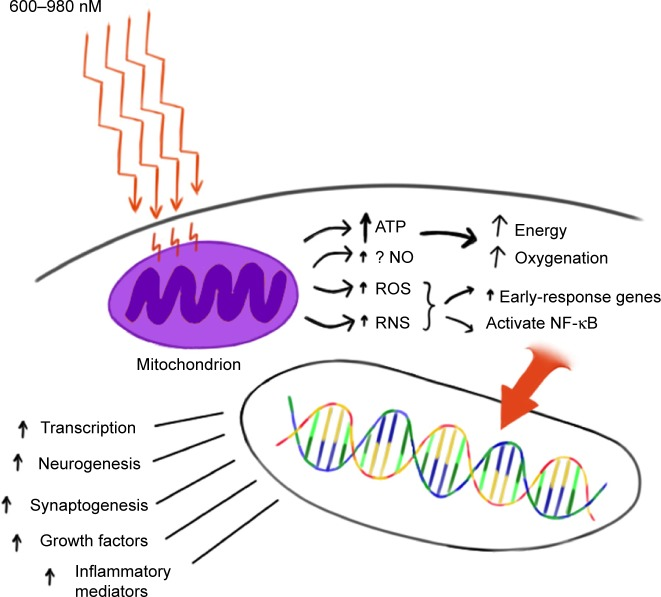
The biology of TLT is fairly well established from photon to mitochondrial cytochrome-C absorption to increased intracellular ATP production. Beyond that, numerous salutory effects are proposed and experimentally can be shown, as the following diagram illustrates.
From http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4550182/figure/f1-ndt-11-2159/
Two clinical studies of TLT serve as guidance for this Phase 1 pilot study. Most recently, a few months ago Henderson et al published the results of a series of 10 patients with TBI and PTSD using HPNIL, showing very impressive PTSD symptom treatment:
DEPRESSION
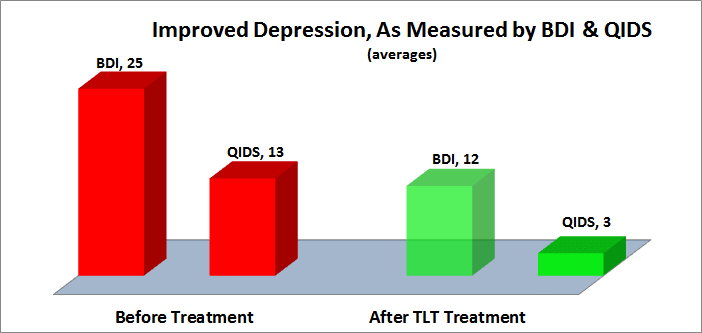
Depression was assessed using both the Quick Inventory of Depressive Symptomatology, QIDS, and the Beck Depression Inventory, BDI.
The QIDS decreased 75% from 13, moderately depressed, to 3, normal. The BDI-II decreased 50% from an average of 25, moderately depressed, to an average of 12, minimal depression. The following chart illustrates the substantive decreased in depression following TLT.
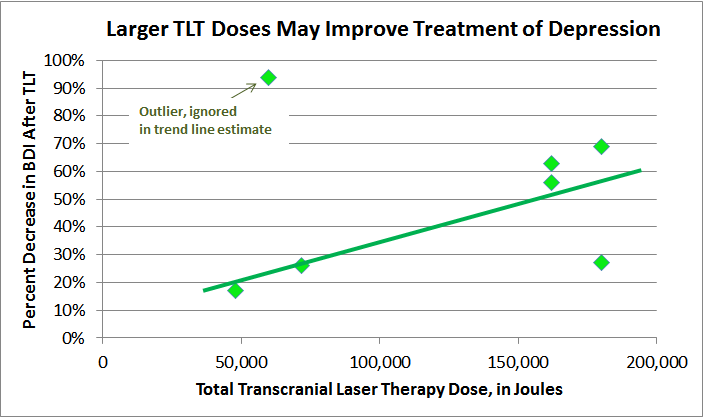
The results from the Denver Protocol, when further analyzed, suggest that improved depression is dose-dependent:
SLEEP DISTURBANCE
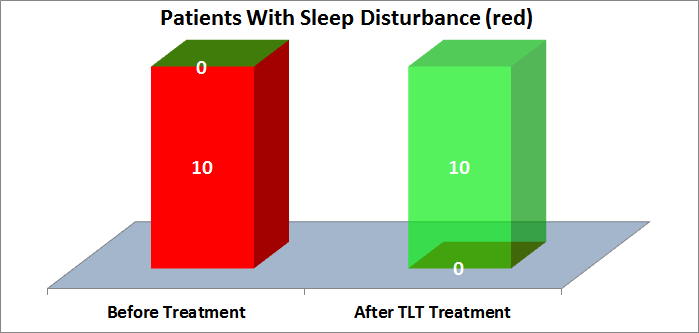
Sleep disturbances, nightmares, and insomnia are PTSD hallmarks. They are also the least likely PTSD symptoms to be treated successfully with current approaches. TLT may represent a breakthrough in treatment of PTSD sleep disturbances. As shown in the graph below, 10 of 10 patients with sleep disturbances reported substantial or total improvements after TLT.
Sleep is the body's natural PTSD salve, helping with the consolidation of fear extinction memory. Nightmares and insomnia interfere with this process. A vicious cycle can occur, with sleep disturbances exacerbating PTSD, which in turn exacerbates sleep disturbances.
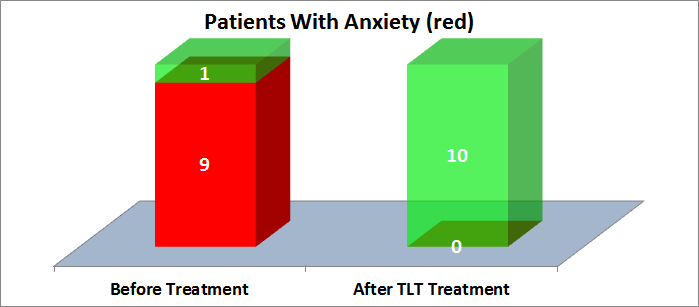
ANXIETY
Anxiety is one of the hallmarks of PTSD. Current PTSD therapies can treat anxiety, and the Denver report indicates that TLT may be one of the best modalities. As shown in the graph below, 9 of 9 patients with anxiety were successfully treated with TLT.
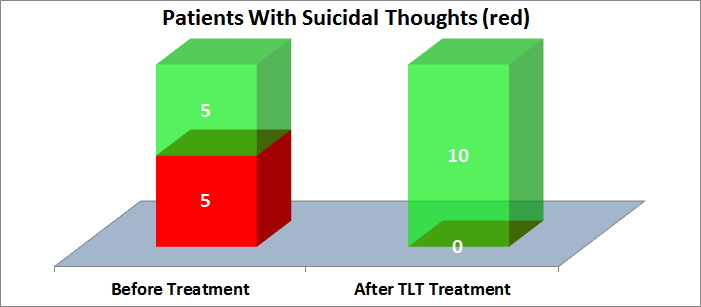
SUICIDE
Statistics from the VA indicate that suicide rates are two times higher for vets than for civilians. Most studies indicate that PTSD puts vets at further risk of suicide. In the Denver report, 5 of 10 patients had suicidal thoughts, and all 5 were successfully treated with TLT, as shown in the chart below.
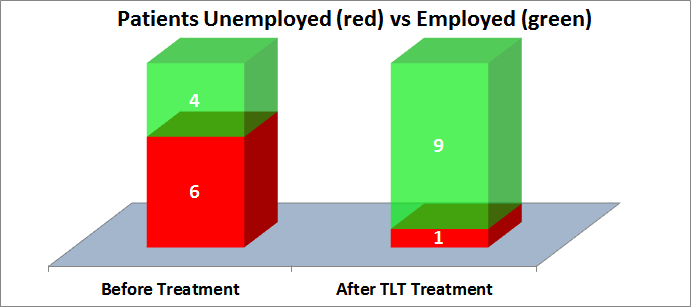
UNEMPLOYMENT
Unemployment is more closely correlated with PTSD severity than depression. Unemployment can be both a cause of worse PTSD symptoms, and the result of PTSD symptoms. In either case, PTSD-related unemployment takes its toll on PTSD patients, families, and society. Impressively, of the 6 patients who were unemployed, 5 of them found gainful employment after TLT, as shown in the following chart.
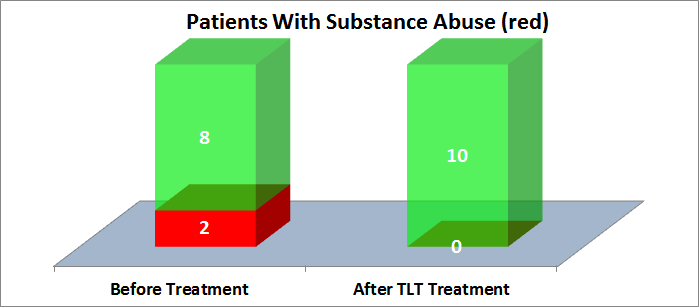
SUBSTANCE ABUSE
Substance abuse is a common comorbidity with PTSD. It can exacerbate, and also result from, PTSD. Two of 10 patients reported substance abuse pre-treatment, and after TLT treatment none reported substance abuse, as shown in the following chart:
While the Denver Protocol showed apparently great treatment results, a 2009 series of NEST studies (NEST-1, NEST-2, NEST-3) failed to demonstrate any beneficial effect of NLT in acute stroke. The precise reasons for failure may be due to NLT's lack of efficacy in acute stroke, but the sub-therapeutic doses of NIL almost certainly played a major or sole factor in the failure. The NIL was applied via a Photothera 2009 5W laser. The laser was applied for just 2 min in 20 locations. Of those 20 locations, only 1 would have been the "correct" stroke location. Working backwards, we can assume 2 min at 1 single location of > 10cm 2 (from the Photothera photo.) So 5W x 120 seconds / 10 cm 2 = 60 J/cm 2 at the scalp in the NEST protocols. Compare that with the Denver protocol of 500 - 1,800 J/cm 2 or the Manhattan Beach protocol of 4,000 J/cm 2 and it is entirely understandable why NEST failed to produce good results.
LLLT
Low level laser therapy (LLLT) is mentioned for clarity. LLLT is usually used when referring to Class 3 lasers which produce less than 0.5 W. In humans, this is sometimes referred to as "Cold Laser" therapy. In humans, LLLT is incapable of penetrating more than 1-2 mm of skin, and therefore incapable of producing clinical results of any kind other than placebo effects. LLLT in animal in vivo models, or as a treatment in in vitro models, is perfectly reasonable. Basically, LLLT is a mouse-sized laser, while HPNIL is a human-sized laser.
Methods
40 volunteer patients, each of whom is a Veteran with PTSD, will be treated with TLT over 1-3 weeks. TLT will be administered with the LiteCure EXP 25 Watt 810/980 nm medical laser, or a Diowave 60 Watt 810 or 980 nm medical laser. Each laser will be set to 15-30 Watts, either continuous wave or 10 Hz Pulsed with a 50% duty cycle. Each session will treat 3 areas (A,B) for 15-30 minutes per area. Sessions will be 1-3 days apart, depending on convenient scheduling. Patients will receive either 3,600 Joules per treatment area per session for 10 sessions (108,000 Joules total) or 10,500 Joules per treatment area per session for 15 sessions (472,500 Joules total). The lower dose is approximately the average total Joules delivered in the Denver protocol. The higher dose is referred to as the "Manhattan Beach protocol."
Skull temperatures will be continuously monitored during treatment using a custom system consisting of two Sierra Olympic Cox Thermography Cameras, model CX640. The custom system will monitor from two angles, and record the temperature changes. A visual and audible alarm will alert of significant rises in temperature. The software and hardware for these alarms be custom built.
Outcome measures will be made at the beginning of the treatment, during the treatment, at the end of the treatment, and for 6 months following treatment. Where possible, these surveys will be made available to patients in secure online web forms. However, it is anticipated that in-office paper forms and postal mail paper forms will also be used and require in-office data entry. Further, it is anticipated that phone follow-up to encourage survey completion after treatment will require a significant effort. See the "Screening Tools & Outcome Measurements" section for a detailed list.
Analysis of all outcome measures will be made and assessed weekly. Analysis will look for treatment outcomes that are correlated with expected variables of dose, frequency, and severity of initial symptoms. In addition, we will analyze data for new potential variables gender, handedness, duration of PTSD, prior treatment modalities, head circumference, BMI, and amount of hair. While 10 patients are too few to get any meaningful data, it is anticipated that with 40 patients that some of these measurements will produce useful insights for future improvement and prediction of treatment improvement.
Inclusion criteria: Age 20-75, Informed consent, documented Veteran, documented PTSD diagnosis from VA or DOD, documented PTSD via pre-trial screening with PCL-5. Initial screening will be done online and via phone interview, followed where appropriate by in-office screening.
Exclusion criteria: Seizure history requiring recent or ongoing medical therapy. Weight < 120 # or > 260 #.
Patients will be offered no financial incentives, and will receive TLT at no cost to them. It is anticipated that many patients will have somatic pain, as historically ⅔ patients with PTSD report significant pain. Treatment of somatic pain with HPNIL, where applicable, will be offered to patients at a 30% discount to standard prices. Additional grants may be sought so that this pain treatment component is offered free of charge to some patients. Pain treatments will be analyzed using the already-included VAS (Visual Analog Scale.)
Screening Tools & Outcome Measurements
The following measures will be analyzed to determine treatment effects: PTSD, suicidal thoughts, sleep disturbance, somatic pain, employment, depression, substance use. Multiple scales and surveys are used to make comparisons to existing and future research easier among a wider number of studies.
|
Area |
Test |
Evaluation Used to Screen for Eligibility? |
Evalua- tion Prior to Study Start? |
Evaluation During Study |
Evaluation At End of Study |
Evaluation After Study Completed |
Available |
|
|
|
|
|
|
|
|
|
|
PTSD |
PCL-5 |
Yes |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
http://www.ptsd.va.gov/professional/assessment/documents/PCL-5_Criterion_A_100313_508.pdf |
|
|
|
|
|
|
|
|
|
|
Pain |
VAS, Visual Analog Scale |
-- |
Yes |
Prior to each TX |
Yes within 1 week |
Monthly for 6 months |
LMDPR form |
|
Pain |
Pain history |
-- |
Yes |
-- |
Yes within 1 week (abbreviated) |
Monthly for 6 months (abbreviated) |
LMDPR form |
|
|
|
|
|
|
|
|
|
|
Depression |
QIDS-SR-16 |
-- |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
http://www.ids-qids.org/translations/english/QIDS-SREnglish2page.pdf |
|
Depression |
BDI-II |
-- |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
http://www.pearsonclinical.com/psychology/products/100000159/beck-depression-inventoryii-bdi-ii.html |
|
|
|
|
|
|
|
|
|
|
Substance Use |
SASSI-3 (TBD) |
-- |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
|
|
Substance Use |
NIDA |
-- |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
http://www.drugabuse.gov/sites/default/files/files/QuickScreen_Updated_2013%281%29.pdf |
|
|
|
|
|
|
|
|
|
|
Employment |
Brief survey to be written |
-- |
Yes |
Weekly prior to TX |
Yes within 1 week |
Monthly for 6 months |
LMDPR to provide |
|
|
|
|
|
|
|
|
|
|
General History |
Med history, PTSD context, treatments tried, meds, pain, service history |
Yes, + PTSD, - Current Seizure TX |
Yes |
-- |
Yes within 1 week (abbreviated) |
Monthly for 6 months (abbreviated) |
LMDPR to provide |
|
General History |
Weight, height - self reported |
-- |
Yes |
-- |
-- |
-- |
LMDPR to provide |
|
|
|
|
|
|
|
|
|
|
Physical |
Head circumference |
-- |
Yes |
-- |
-- |
-- |
LMDPR to provide |
|
Physical |
Hair pics |
-- |
Yes |
-- |
-- |
-- |
LMDPR to provide |